Health and wellness
Vitamin C and cancer chemoprevention: reappraisal
Several studies have reported that even a moderate daily dose of supplementary vitamin C (200 mg) induces the formation of genotoxins from lipid hydroperoxides, thereby resulting in DNA damage and initiation of carcinogenesis. However, other reports questioned the experimental designs used and suggested that the chemopreventive effects of vitamin C may be linked to the inhibition of tumor promotion as well as to the blocking of tumor initiation. In this article, we discuss issues of contention and some controversies related to the potential chemopreventive effects of vitamin C in carcinogenesis.
Creative in All Aspects
Over the past 3 decades, extensive efforts were made to treat cancer. However, as recent statistics show (Figure 1), the incidence of and the mortality from cancer have in general not diminished but have instead increased (1, 2). Therefore, more attention is now focused on prevention as the ultimate strategy for management of cancer, even though advances in cancer treatment could offer considerable benefits to many patients. Epidemiologic and laboratory studies indicate that a high consumption of antioxidant-rich fruit and vegetables can reduce the risk of cancer (3–6). In the past several years, antioxidative vitamins and phenolic substances derived from the daily diet received considerable attention because of their potential chemopreventive activities.
Figure 1

Vitamin C is considered to be one of the most prevalent antioxidative components of fruit and vegetables, and it could exert chemopreventive effects without apparent toxicity at doses higher than the current recommended dietary allowance of 60 mg/d (7). It has also been used as a dietary supplement intended to prevent oxidative stress–mediated chronic diseases such as cancer, cardiovascular disease (8), hypertension (9), stroke (10), and neurodegenerative disorder (11). Currently, the US Department of Agriculture and the National Cancer Institute recommend the consumption of a minimum of 5 servings of fruit and vegetables/d to prevent cancer (7). Thus, should these recommendations be followed, a daily consumption of 200–280 mg vitamin C might be achieved. Although it has generally been acknowledged that vitamin C protects cells from oxidative DNA damage, thereby blocking the initiation of carcinogenesis, some studies have shown that dietary vitamin C supplementation is not beneficial but, rather, may cause DNA damage. These findings suggest that dietary components other than vitamin C may play an important role in cancer prevention. Moreover, the chemopreventive mechanism of vitamin C may be linked to the inhibition of other processes—in particular, tumor promotion—rather than to that of tumor initiation. In this article, we discuss the multiple mechanisms underlying the chemopreventive effects of vitamin C and the current controversies about the beneficial or detrimental effects of this dietary antioxidant.
VITAMIN C AND CANCER
Recently, Lee et al (12) reported that 200 mg vitamin C/d, which can be obtained from the daily diet, induced the decomposition of lipid hydroperoxides to endogenous genotoxins, which suggested that vitamin C may not be effective in cancer chemoprevention. However, their experiment was based only on in vitro chemical reactions. The concentration of lipid hydroperoxides used in the study (400 μmol/L) is not physiologically relevant, because concentrations in human blood range only from 10 to 500 nmol/L (13, 14). Moreover, in the human body, the probability, if any, of the induction of such adverse effects by a daily intake of dietary vitamin C would be very low because of the presence in cells of endogenous antioxidants and antioxidant enzymes such as glutathione peroxidase and catalase. According to a recent report by Lenton et al (15), the consumption of 500–1000 mg vitamin C/d as a supplement augments glutathione in human lymphocytes, which may be associated with the inhibition of lipid peroxidation. A report by Levine et al (16) also pointed out that studies with human volunteers were not sufficient to support the notion of vitamin C–induced lipid peroxidation. In addition, vitamin C is capable of regenerating vitamin E from the α-tocopheroxyl radical, which is formed as a result of the inhibition of lipid peroxidation by vitamin E and which has additional well-defined biological functions, including the cofactor activity for several important enzymatic reactions.
On the basis of an increased number of modified DNA basis in lymphocytes, Podmore et al (17) claimed that dietary supplementation with vitamin C at 500 mg/d exerts prooxidant and mutagenic effects in humans. Although 8-oxoadenine concentrations increased, Podmore et al also found a significant decrease in 8-oxoguanine concentrations. Because 8-oxoguanine is considered to be the important mutagenic lesion in DNA, these data indicate that vitamin C can protect DNA from potential mutagenic alterations. Lenton et al (18) found that intracellular vitamin C concentrations were negatively correlated with 8-oxo-deoxyguanosine concentrations in lymphocytes from 105 healthy volunteers. In another report, 8-oxo-2`-deoxyguanosine concentrations in mononuclear cell DNA, serum, and urine from subjects undergoing supplementation with 500 mg vitamin C/d also decreased, and this result was strongly correlated with increases in plasma vitamin C concentrations (19). In addition, vitamin C was found to be protective against DNA damage induced by hydrogen peroxide in human lymphocytes (20). However, the relevance of oxidative DNA base modification as a biomarker of carcinogenesis is being questioned because of a frequent artifact in measuring 8-oxo-deoxyguanosine concentrations in DNA (21, 22). Moreover, there is no consistency in actual 8-oxo-deoxyguanosine concentrations in human DNA (21, 22). With the above problems taken into consideration, Lutsenko et al (23) recently used a quantitative plasmid-based genetic system to show that vitamin C can prevent hydrogen peroxide–induced mutations in human cells. Those investigators suggested that high intracellular vitamin C concentrations could decrease the DNA mutations caused by oxidative stress in humans.
Vitamin C can exert a prooxidant activity under certain conditions, particularly in the presence of transition metal ions or alkali. Thus, vitamin C in vitro reduces free ferric iron that generates hydrogen peroxide in the Fenton reaction and results in the production of hydroxyl radicals. The reactive hydroxyl radical quickly reacts with critical cellular macromolecules, including DNA, which may lead to mutagenesis and the initiation of cancer. However, the amounts of free transition metals in vivo are very small because they efficiently bind to proteins. Other studies showed that the consumption of 500 mg supplemental vitamin C/d did not result in significant oxidative DNA damage (24). Even a 5000-mg intake of vitamin C was shown not to promote cancer or induce DNA damage (25). Moreover, vitamin C was found to predominantly reduce oxidative damage in vivo even in the presence of iron (26). Although extensive oxidative stress can certainly cause oxidative damage, moderate amounts of reactive oxygen intermediates (ROIs) can serve as a second messenger in the intracellular signaling cascades. Several antioxidant phytochemicals can induce phase II detoxification of antioxidant enzymes by triggering nuclear translocation of the transcription factors such as NF-E2–related factor 2 (Nrf2) or nuclear factor-κB and their subsequent binding to antioxidant response element and κB binding element, respectively (27). It is interesting to note that many of the inducers capable of activating these transcription factors mimic prooxidants and electrophiles, although most of them are antioxidants by nature. Therefore, it would worthwhile to examine whether vitamin C can induce the expression of phase II detoxification or antioxidant enzymes via its prooxidant potential.
Early epidemiologic evidence indicated that high intakes of vitamin C–rich fruit and vegetables and a high vitamin C concentrations in serum are inversely associated with the risk of some cancers. In 1991, Henson et al (28) analyzed 46 epidemiologic studies on the protective effects of vitamin C against various types of cancers; 33 of these studies found a significant link between vitamin C intake and a reduced incidence of cancer. A more recent analysis by Carr et al (26) showed that vitamin C acts as an antioxidant in vivo. Of the 44 published in vivo studies examined, 38 found a decrease in the number of markers of oxidative damage to DNA, lipid, or protein; 14 showed no changes; and only 6 reported an increase in oxidative damage after supplementation with vitamin C. A recent epidemiologic study by Khaw et al (8) showed that a high vitamin C concentration in plasma had an inverse relation with cancer-related mortality. In 1997, expert panels at the World Cancer Research Fund and the American Institute for Cancer Research estimated that vitamin C can reduce the risk of the stomach, mouth, pharynx, esophagus, lung, pancreas, and cervical cancers (7).
According to most information in the literature, the high consumption of vitamin C–rich fruit and vegetables is not likely to be harmful. In general, data from in vitro and in vivo experiments and population-based studies do not indicate that high doses of vitamin C are linked to increased oxidative DNA damage or an elevated risk of cancer. Although the results of the previous studies are not completely consistent, in most instances, they do provide support for the notion that vitamin C intake may decrease cancer risk. However, the clinical trials based on a high dose of dietary vitamin C supplement are not supportive (29, 30), although epidemiologic and observational studies based on food intake provide evidence for a strong, protective role of vitamin C against cancer (6, 28).
PLAUSIBLE CHEMOPREVENTIVE MECHANISMS OF VITAMIN C
Antioxidant effects
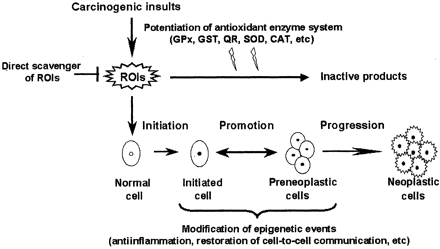
How can vitamin C prevent cancer? Although vitamin C has been known to stimulate immune function, inhibit nitrosamine formation, and block the metabolic activation of carcinogens, its cancer-preventive effects may be associated mainly with its protective effects against oxidative stress. ROIs are major molecules that can cause cancer through multiple mechanisms. The carcinogenic effect of oxidative stress has been primarily focused on the genotoxicity of ROIs, and vitamin C can protect against oxidative DNA damage, which is implicated in tumor initiation. ROIs have also been known to play a significant role in the promotional stage of carcinogenesis. In particular, several oxidants and free radical generators are tumor promoters. A theory (31–33) of epigenetics posits that greater attention must be paid to those processes that do not involve DNA damage in multistage carcinogenesis. The promotional phase of carcinogenesis is a consequence of epigenetic events involving inflammation and the inhibition of gap junction intercellular communication (GJIC), which could be mediated by ROIs (31, 32, 34–38). Besides the antioxidant activity, an intrinsic prooxidant potential of vitamin C may contribute to its chemopreventive properties. As described in the previous section, low or baseline oxidative stress appears to be essential for cellular signal transduction that leads to the induction or potentiation of some detoxification or antioxidant enzyme systems. Antioxidant micronutrients may act as mild prooxidants to supply limited amounts of ROIs whenever needed for triggering the antioxidant signal transduction. If this is the case, it remains to be clarified when and how the prooxidant activity of vitamin C is turned on in the intracellular redox milieu at the same time that vitamin C is fighting, as an antioxidant, against excess oxidative stress.
Antiinflammatory activity
There is considerable evidence that ROIs are somehow involved in chronic inflammation and cancer (31, 34–36). The generation of oxidative stress is an integral part of the inflammatory response associated with tumor promotion. Thus, many compounds with antioxidant capability can inhibit tumor promotion and inflammation (31, 34, 35). Several studies showed strong evidence that gastric cancer is a consequence of chronic inflammation; on the basis of this evidence, the inflammatory process caused by the overproduction of ROIs could be a target of vitamin C (39). Vitamin C indeed was shown to reduce inflammation caused by ROIs, thereby attenuating gastric cancer (39). A recent human study also showed low vitamin C concentrations in gastric juice in the earlier stage of carcinogenesis (40). The protective effects of vitamin C against gastric carcinogenesis may be partly related to the scavenging of the mucosal oxygen radicals (41) and to the inhibition of the formation of carcinogenic nitrosamines. However, in a recent report, treatment of endothelial cells with vitamin C resulted in the accumulation of a large amount of this antioxidant inside the cells, which consequently decreased both the intracellular oxidant status and inducible nitric oxide synthase induction (42). Vitamin C was also shown to inactivate nuclear factor-κB in endothelial cells during the inflammation process, independently of its antioxidant activity (43). Therefore, the antiinflammatory activity of vitamin C may be mediated by multifactorial mechanisms, which are not necessarily associated with its intrinsic antioxidant activity.
Restoration of cell-to-cell communication
Cell-to-cell communication through gap junction channels is essential for maintaining homeostatic balance through modulation of cell proliferation and differentiation in multicellular organisms (44). Inhibition of cell-to-cell communication is strongly related to the carcinogenic process, particularly to tumor promotion (32, 45). Hydrogen peroxide, a well-known tumor promoter, also inhibits GJIC (38). Recently, we reported that vitamin C exerts protective effects against the disruption of GJIC by hydrogen peroxide (46). However, antioxidants such as propylgallate and Trolox (Sigma Chemical Co, St Louis) did not prevent the hydrogen peroxide–mediated inhibition of GJIC (38). Furthermore, vitamin C at a concentration that restored the dysregulated GJIC did not destroy the hydrogen peroxide when a direct measurement was made by using the ferrous ion oxidation–xylenol orange assay (HJ Lee and CY Lee, unpublished observations, 2000). In this respect, the effect of vitamin C on GJIC appears to be related to a different mechanism, such as the inhibition of signal transduction (45, 47).
An analysis by Rosenkranz et al (48) found that the inhibition of GJIC is strongly linked to the carcinogenic process, a biological phenomenon that may involve the inflammatory process, and to developmental effects in rodents. Integration of the analysis also suggests that the inhibition of GJIC is involved in nongenotoxic cancer induction and tumor promotion (48). Therefore, we suggest that the chemopreventive effects of vitamin C in carcinogenesis may be linked to the protective effects of vitamin C against epigenetic mechanisms, such as the inflammation and inhibition of GJIC, as well as to antioxidant activities (Figure 2).
Figure 2
VITAMINS, PHYTOCHEMICALS, DIETS, AND CANCER PREVENTION
Although a recent study showed an inverse relation between plasma vitamin C and cancer mortality (8), similar results of lower cancer mortality were observed in another study that involved daily fruit intake (49). Population-based studies still show that a low risk of cancer is more closely related to antioxidant-rich whole diets than to individual dietary antioxidants. These results imply that diet as a whole plays a more important role than do individual components. The cancer-preventive effects of vegetables and fruit may result from multiple combined effects of various phenolic phytochemicals, vitamins, dietary fibers, indoles, allium compounds, and selenium rather than from the effect of a single active ingredient. In particular, as addressed in our reports and reviews (31, 34, 35, 46, 50–52), many dietary phenolic phytochemicals may have stronger antioxidant and antitumor promotion effects than do antioxidant vitamins, which may contribute to the chemopreventive effects of the phytochemicals in carcinogenesis. We previously confirmed that most beneficial effects of apples on carcinogenesis may be due not to the presence of vitamin C alone but to the synergism elicited by various phenolic ingredients (46, 50, 51). Furthermore, an epidemiologic study suggested that the inverse relation between the consumption of antioxidant-rich diets and the incidence of cancer has to do with the intake of flavonoids rather than with that of vitamins (53, 54). These results suggest that the chemopreventive effects of vegetables and fruit in carcinogenesis may result from various phenolic phytochemicals rather than from vitamins.
Conclusions
Although elimination or minimization of exposure to diverse environmental carcinogens may be a strategy for preventing most human cancers, complete avoidance of exposure to cancer-initiating factors may be unrealistic. Because tumor promotion is closely linked to oxidative and inflammatory processes and because it is a relatively long and reversible process, antioxidant-rich whole foods such as fruit, vegetables, and grains can efficiently reverse and suppress the carcinogenic process. Thus, the consumption of 5 servings of fruit and vegetables containing 200–280 mg vitamin C/d could be still recommended, although further research is needed to establish whether vitamin C supplementation beyond normal dietary intake is beneficial.
Each author contributed jointly, equally, and cooperatively to this work. None of the authors had any conflicts of interest, either personal or financial, with regard to this study
Subsribe To Our Newsletter
Stay in touch with us to get latest news and special offers.
Address
1603 BABCOCK RD STE 238, SAN ANTONIO, TX 78229
Call Us
+1 (210)-384-2988
Email Us
sales@ingramformulations.com
